Abstract
Introduction PD-1 is a negative costimulatory receptor on activated T lymphocytes which counters the activation signal provided by T cell receptor ligation. The expression of PD-1 on T lymphocytes can be dynamically modulated by DNA methylation. A recent study in solid tumors (Kleffel S et al, Cell 2015) suggested that PD-1 was not only expressed by immune cells, but also by a subpopulation of cancer cells, and the cancer intrinsic PD-1 can promote tumorigenesis. Our previous study (Yang H et al, Leukemia 2014) demonstrated an increased PD-1 mRNA expression in peripheral blood mononuclear cells from MDS patients under hypomethylating agent (HMA) treatment, but little is known about detailed PD-1 expression in MDS. PD-1 signaling has been reported to be involved in MDS pathogenesis and resistance mechanisms to HMAs (Yang H et al, Leukemia 2014). Therefore, the PD-1 pathway represents an attractive target and anti-PD-1 monoclonal antibodies are being increasingly used to study MDS. Precise understanding of PD-1 expression in MDS patients may allow for effective treatment.
Methods and Human Specimens With this purpose, using multicolor flow cytometry analysis, we studied the PD-1 expression on bone marrow Lin-CD34+ cells (Lineage cells: CD2, CD3, CD4, CD7, CD10, CD11b, CD14, CD19, CD20, CD33, CD56 and CD235a) from 51 patients enrolled in a clinical trial 2014-0930. Based on 5-azacytidine treatment, these patients can be divided into two groups: group A included 29 patients (57%) (22 MDS, 5 CMML, 1 MDS/MPD and 1 AML) which were treated with 5-azacitidine in combination with ipilimumab (N=10), nivolumab (N=16) or ipilimumab + nivolumab (N=2), one patient treated with 5-azacytidine only was also included; group B included 22 patients (43%) (16 MDS, 4 CMML and 2 MDS/MPD) which did not receive 5-azacytidine treatment and were treated with ipilimumab (N=12), nivolumab (N=5) or ipilimumab + nivolumab (N=5) alone. Positive PD-1 expression was considered when the median fluorescence intensity (MFI) ratio between sample and FMO negative control was more than 2.
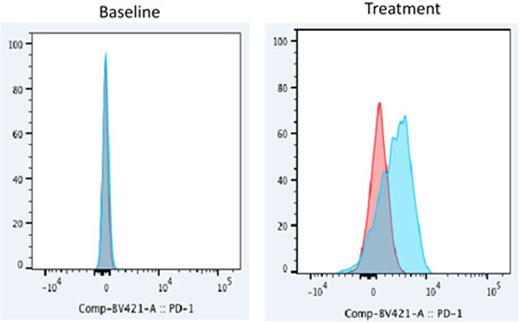
Results In group A, 26 patients (90%) were previously untreated at the time of enrollment. PD-1 expression was not observed in 8 untreated baseline samples collected. We then performed the analysis in samples under different time points of treatment. PD-1 expression was observed in 11 patients (38%, 9 responders, 1 stable disease and 1 non-responder) in at least one time point during treatment. Interestingly, 9 (82%) of them were treated with 5-azacytidine in combination with nivolumab, the other two were treated with 5-azacytidine in combination ipilimumab and ipilimumab + nivolumab separately. PD-1 expression was observed in 56% of the patients under 5-azacytidine in combination with nivolumab treatment. Figure 1 is an example of PD-1 expression in Lin-CD34+ cells from patient treated with 5-azacytidine in combination with nivolumab.
In group B, all patients except one (95%) were previously treated at the time of enrollment. PD-1 expression was observed in 2 out of 13 baseline samples collected, and the PD-1 expression was not detected on these two patients after follow-up treatment with ipilimumab. PD-1 expression was observed in two patients (9%, 1 responder, 1 stable disease) in at least one time point during treatment (1 ipilimumab, 1 ipilimumab + nivolumab).
Taken together, PD-1 expression was significantly induced in patients treated with 5-azacytidine when compare to patients without 5-azacytidine treatment (38% vs 9%, p=0.023), especially in the group of patients treated with 5-azacytidine in combination with nivolumab (56% vs 10%, p=0.037, compare with 5-azacytidine in combination with ipilimumab). Induction of PD-1 expression may contribute to the response of the treatment (77% vs 46%, p=0.058).
Conclusion Our study demonstrates that induced expression of PD-1 on MDS Lin-CD34+ cells may contribute to the response of MDS patients to treatment with 5-azacytidine in combination with nivolumab. PD-1 methylation as well as other biological functions related to this treatment need to be further studied.
Colla:Abbvie: Research Funding. Daver:Novartis: Research Funding; Incyte: Research Funding; Otsuka: Consultancy; Daiichi-Sankyo: Research Funding; Sunesis: Consultancy; ImmunoGen: Consultancy; Karyopharm: Consultancy; Alexion: Consultancy; Incyte: Consultancy; Kiromic: Research Funding; Pfizer: Consultancy; ARIAD: Research Funding; Pfizer: Research Funding; Novartis: Consultancy; BMS: Research Funding; Karyopharm: Research Funding; Sunesis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal